Volume 19, Issue 2 (Mar-Apr 2025)
mljgoums 2025, 19(2): 10-14 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Konikkara K P, Mukundan A, John R. SARS-CoV-2 IgG antibody response to a viral vector vaccine among health care workers in a tertiary care hospital. mljgoums 2025; 19 (2) :10-14
URL: http://mlj.goums.ac.ir/article-1-1663-en.html
URL: http://mlj.goums.ac.ir/article-1-1663-en.html
1- Department of Microbiology Government Medical College Thrissur, Kerala, India , kavithageejo@gmail.com
2- Department of Microbiology Government Medical College Thrissur, Kerala, India
2- Department of Microbiology Government Medical College Thrissur, Kerala, India
Full-Text [PDF 386 kb]
(144 Downloads)
| Abstract (HTML) (430 Views)
The baseline characteristics analyzed included age, gender, co-morbidities, area of work and BMI. Different categories of health-care workers were included in this study. Other parameters included were gap between vaccination and estimation of antibody titer as well as history of previous COVID-19 infection (Table 3).
The association between various factors and antibody titer was assessed (Table 4).
No significant correlation of antibody response was observed with age, gender, comorbidities, area of work (Whether worked in COVID ICUs and wards) and BMI (p-value>0.05).
The antibody response was found to be statistically significant among healthcare workers in various categories (p-value <0.001).
Antibody titer level was high within 3 months of vaccination and waning of titer was noticed as months passed by. This was also found to be significant. (p-value<0.001)
Those who had been previously infected with SARS-CoV-2 produced significantly higher antibody titer than those who were never infected. (p-value<0.001).
Discussion
In the current cross-sectional study conducted among 187 health-care workers, who were administered two doses of Covishield vaccine, 99·47% developed Anti-SARS-CoV-2 antibodies including IgG against RBD protein. The seroconversion rates of most of the studies were greater than 95%. Hoque et al reported 100% of antispike protein IgG antibody (7). Seropositivity rate obtained in a study done by Singh et al was 98.1 % (4). Another study revealed 96.6% antibody response in their participants (8). Lower seropositivity (69.9%, 69.67%) was observed in the studies conducted by Mahadevaiah et al, Njarekkattuvalappil et al respectively (9,10).
Age, gender, comorbidities, area of work, body mass index, history of previous COVID infection, gap between vaccination and estimation of antibody titer were assessed as variables affecting antibody response.
No significant difference in antibody titer was seen in relation to age, gender, BMI, area of work and comorbid conditions. Singh et al obtained similar inference with regard to age, sex, BMI, and comorbidities (4). Balasubramanian et al couldn’t find significant association with age, sex and BMI (11). As per Njarekkattuvalappil et al BMI, gender and comorbidities were not found to be statistically significant (10). In contrast to the interpretation we made, a study done by Lustig et.al found that antibody responses were different in health care workers of varying age, gender, and comorbid conditions (12).
There is enough evidence that older individuals have reduced immune response (2,7,10,13,14). We were unable to ascertain this reality. Perhaps the very small number of participants over 60 in this cohort is the reason for this.
According to Uysal et al, the titers of anti-RBD antibodies were lower in obese participants compared to normal-weight participants. Excessive adiposity might have a negative impact on the immune system. Another presumed finding is that, as the ACE2 receptor is markedly seen in adipose tissue, obese individuals are more prone to get infection (15).
Similar to our findings, comorbidity did not make any difference in the SARSCoV-2 Ig G antibody level in other studies too (4,10,16). Since the health-care workers who participated in the study were with manageable comorbid conditions, we could not infer the association between antibody response and comorbidities.
In our study, different categories of health-care workers showed a significant correlation with the antibody response. The geometric mean titer was higher for nurses and other hospital staff compared to doctors. It could be due to the fact that a greater number of participants in these groups probably had asymptomatic infection with SARS COV-2. No difference in seroprevalence between various groups of health care workers were observed in serosurveillance done by Murhekar (17). Only limited studies are available regarding the correlation between post vaccination SARS-CoV2 antibodies and different categories of health-care workers.
The antibody titers of health-care workers who had previously been infected with SARS-CoV-2 were higher than those who were never infected; the association was found to be statistically significant. There are also other researchers who support our findings (11,16,18). The high antibody levels in the previously infected groups may be due to the fact that B cells create antibodies which multiply after each exposure, whether due to an infection or vaccination (18). Humans who have been infected with SARS-CoV-2 tend to produce long-lived bone marrow plasma cells, and the levels of serum anti-SARS-CoV-2 spike protein (S) antibodies remain detectable as long as 11 months post infection (19).
The level of antibodies in our participants decreased over time and their antibody titers were maximum within three months of vaccination. Waning of antibody responses to vaccines needs to be augmented by booster doses. Some other studies also noticed a decrease in antibody titer as time passed by (16,20,21). It is advisable that a follow-up study be conducted to determine the optimal timing for administration of booster dose to the previously infected group, by performing a regular serological analysis and monitoring the decline in antibody levels over time. The resulting data will help in developing strategies for vaccination as well as for developing protocols to prioritize individuals for booster shots (18).
Regarding limitations of this study, immunity against vaccination depends upon Neutralizing antibody titer and cell-mediated immune response which were not assessed. Effectiveness of booster dose could not be assessed.
Conclusion
There was a robust immune response in vaccine recipients irrespective of their age, gender, BMI, and comorbidity status. We could assess the antibody response after vaccination for different periods ranging from 1month to 9 months. SARS-CoV-2-infected healthcare workers had higher antibody titer than those who were not infected. Future studies are desirable to determine the protective level of antibody titer.
Acknowledgement
We would like to extend heartfelt gratitude to all the health-care workers of our hospital who took part in this study.
Funding sources
The authors received financial support from State board of medical research, under the head of account 2210-05-105-99-34-OC (Plan).
Ethical statement
The study was approved by the institutional Ethics committee (Reference no.IEC/GMC TSR/176/2021), Government Medical College, Thrissur, Kerala India.
Conflicts of interest
All authors declare no conflict of interest.
Author contributions
Concept and design of study were done by Kavitha Paul Konikkara and Reena John. Data collection was by Kavitha Paul Konikkara and Aiswarya Mukundan. Data analysis and drafting of the manuscript were carried out by Kavitha Paul Konikkara and Aiswarya Mukundan. Review and editing were by Reena John. Final approval of the version to be published were by Kavitha Paul Konikkara, Aiswarya Mukundan and Reena John.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Full-Text: (50 Views)
Introduction
COVID-19 cases associated with the SARSCoV-2 virus were first reported from Wuhan, Hubei province in China, in December 2019. COVID-19 was declared as a global pandemic on March 11, 2020. Globally, as of 23rd May 2022, 525,467,084 confirmed cases of COVID-19 have been reported to World health organization including 6,285,171 deaths (1). Health- care workers are at higher risk of developing COVID-19 infection as they are front-line workers during pandemics. Vaccination seems to be the only effective means of curtailing the infection. A variety of COVID-19 vaccines were developed within a year, after the successful completion of phase 3 trials, which enabled them to be used in mass vaccination campaigns worldwide. As soon as phase 3 trials were completed, several types of COVID-19 vaccines were developed and deployed in numerous countries around the world for use in mass immunization campaigns (2). Kerala was the first state in India to be infected with COVID-19, and Thrissur district confirmed the first case of Coronavirus on 30th January 2020 (3). Our institute managed this case successfully by taking all the precautions. After receiving Emergency Use Approval (EUA), vaccination in India began on January 16, 2021 (4). Likewise, our tertiary care center conducted the vaccination program for health-care workers with a two-dose regimen of the ChAdOx1 nCoV19 coronavirus vaccine (Covishield), given intramuscularly at four to six weeks interval. The Covishield vaccine (ChAdOx1-nCOV or AZD1222, obtained from Oxford University and AstraZeneca, developed by Serum Institute of India in Pune) is a chimpanzee adenovirus-vectored vaccine with the SARS-CoV-2 spike antigen encoded in genetically modified human embryonic kidney cells (HEK-293) manufactured at Serum Institute of India in Pune.4 With two vaccination doses, the spike protein induced very high antibody titer, especially in people with prior SARS-COV2 infection (5).
Despite advances in the field, there is still lack of information regarding how much and how long these novel vaccines can elicit a response, both on the humoral and cellular levels (4). So far, little is known about the presence of SARS COV-2 antibodies of health care workers in Kerala. The Chemiluminescence immunoassay was found to be superior to ELISA in detecting antibodies by Lin et al (6). Hence the present study was undertaken to estimate the antibody titer among vaccinated health care workers by Chemiluminescence immunoassay. The effect of various factors (Age, gender, comorbidities, body mass index, area of work and history of prior COVID infection) on antibody response was also evaluated.
Methods
The study was conducted between November 2021 and January 2022 at Government Medical College, Thrissur after obtaining consent from The Institutional Ethics Committee (Reference no. IEC/GMC TSR/176/2021). It was a Cross-sectional study and the study population comprised of the health-care workers in the hospital. All categories of staff who were vaccinated with two doses of ChAdOx1 nCoV19 coronavirus vaccine (Covishield) from January 2021 to October 2021 with or without SARS-CoV-2 infection were included in the study and those who were not willing to participate in the study and persons with contraindications for venipuncture were excluded.
Sample size
The sample size was calculated as per the study conducted in Gujarat by Awadhesh Kumar Singh4, using the formula where the Median [IQR] antispike antibody titer was 127.0 with a IQR of [80.5-268.5]. Approximate Standard deviation was calculated from the IQR as SD=IQR/1.35.Thus the SD was taken as 139.25 with a d of 20 and alpha error of 5% and the minimum sample size was found to be 187.
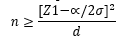
Methodology
List of health care workers were obtained from the Superintendent and the Principal. From the list, health care workers were selected randomly by using a random number generator. Those who were willing to participate were explained about the study purpose and informed consent was taken before the study. The subjects were provided with a proforma to obtain clinical and demographic information, details of COVID-19 testing and vaccination.
The study subjects were directed to the blood collection room and 3ml venous blood was drawn from each health-care worker in a sterile plain vacutainer under strict aseptic precautions. Serum was separated and stored at -20 °C until analysis. Quantitative determination of antibody was done by chemiluminescence immunoassay as per the manufacturer’s instructions, in a National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited lab.
The Elecsys Anti-SARS- CoV-2 assay was performed on Cobas e 411, e 601 and e 602 analyzers (Roche Diagnostics, Mannheim, Germany) for the in vitro quantitative determination of antibodies (Including IgG). The test principle was double antigen sandwich assay which used a recombinant protein representing the Receptor Binding Domain of the S antigen. In brief, the sample was incubated with the biotinylated recombinant antigen and the recombinant antigen labelled with ruthenium. This formed a sandwich complex. In the next step, streptavidin coated microparticles were added and the complex became bound to solid phase. The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of an electrode. Application of a voltage to the electrode induced chemiluminescent emission which was measured by a photomultiplier. Results were determined by a calibration curve and the analyte concentration of each sample was expressed in U/ml. Measuring range spanned from 0.40-250 U/ml. Values greater than or equal to 0.8 U/ml were considered positive and less than 0.8U/ml were considered negative. The sensitivity and specificity of the kit are 98.8% and 99.9 % respectively as per the manufacturer.
Numerical variables were expressed as mean and standard deviation. Categorical variables were expressed as frequency and percentage. The log transformed Antibody titer data were presented as geometric mean with 95% confidence level. Unpaired t test was utilized to assess two groups and ANOVA test was used to compare the differences among more than two group’s data on Antibody titer. The data was entered into Microsoft excel and analysed by using statistical software IBM SPSS Version 25. The p-value < 0.05 was considered as statistically significant.
Results
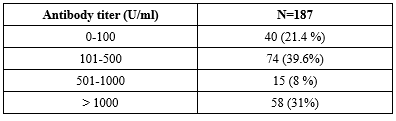
The study enrolled 187 health care workers who met the eligibility criteria. The data of 187 participants who received two doses of Covishield vaccine were included in the final statistical analysis. Of the 187 participants, 186 (99.47%) showed detectable Anti-SARS-CoV-2 antibody levels against the RBD protein. Values greater than or equal to 0.8 U/ml were considered positive and less than 0.8U/ml were considered negative. Lowest antibody titer was found to be 0.6U/ml and highest antibody titer was 5521U/ml. Table 1 shows mean, median and standard deviation of the total antibody level.
COVID-19 cases associated with the SARSCoV-2 virus were first reported from Wuhan, Hubei province in China, in December 2019. COVID-19 was declared as a global pandemic on March 11, 2020. Globally, as of 23rd May 2022, 525,467,084 confirmed cases of COVID-19 have been reported to World health organization including 6,285,171 deaths (1). Health- care workers are at higher risk of developing COVID-19 infection as they are front-line workers during pandemics. Vaccination seems to be the only effective means of curtailing the infection. A variety of COVID-19 vaccines were developed within a year, after the successful completion of phase 3 trials, which enabled them to be used in mass vaccination campaigns worldwide. As soon as phase 3 trials were completed, several types of COVID-19 vaccines were developed and deployed in numerous countries around the world for use in mass immunization campaigns (2). Kerala was the first state in India to be infected with COVID-19, and Thrissur district confirmed the first case of Coronavirus on 30th January 2020 (3). Our institute managed this case successfully by taking all the precautions. After receiving Emergency Use Approval (EUA), vaccination in India began on January 16, 2021 (4). Likewise, our tertiary care center conducted the vaccination program for health-care workers with a two-dose regimen of the ChAdOx1 nCoV19 coronavirus vaccine (Covishield), given intramuscularly at four to six weeks interval. The Covishield vaccine (ChAdOx1-nCOV or AZD1222, obtained from Oxford University and AstraZeneca, developed by Serum Institute of India in Pune) is a chimpanzee adenovirus-vectored vaccine with the SARS-CoV-2 spike antigen encoded in genetically modified human embryonic kidney cells (HEK-293) manufactured at Serum Institute of India in Pune.4 With two vaccination doses, the spike protein induced very high antibody titer, especially in people with prior SARS-COV2 infection (5).
Despite advances in the field, there is still lack of information regarding how much and how long these novel vaccines can elicit a response, both on the humoral and cellular levels (4). So far, little is known about the presence of SARS COV-2 antibodies of health care workers in Kerala. The Chemiluminescence immunoassay was found to be superior to ELISA in detecting antibodies by Lin et al (6). Hence the present study was undertaken to estimate the antibody titer among vaccinated health care workers by Chemiluminescence immunoassay. The effect of various factors (Age, gender, comorbidities, body mass index, area of work and history of prior COVID infection) on antibody response was also evaluated.
Methods
The study was conducted between November 2021 and January 2022 at Government Medical College, Thrissur after obtaining consent from The Institutional Ethics Committee (Reference no. IEC/GMC TSR/176/2021). It was a Cross-sectional study and the study population comprised of the health-care workers in the hospital. All categories of staff who were vaccinated with two doses of ChAdOx1 nCoV19 coronavirus vaccine (Covishield) from January 2021 to October 2021 with or without SARS-CoV-2 infection were included in the study and those who were not willing to participate in the study and persons with contraindications for venipuncture were excluded.
Sample size
The sample size was calculated as per the study conducted in Gujarat by Awadhesh Kumar Singh4, using the formula where the Median [IQR] antispike antibody titer was 127.0 with a IQR of [80.5-268.5]. Approximate Standard deviation was calculated from the IQR as SD=IQR/1.35.Thus the SD was taken as 139.25 with a d of 20 and alpha error of 5% and the minimum sample size was found to be 187.
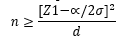
Methodology
List of health care workers were obtained from the Superintendent and the Principal. From the list, health care workers were selected randomly by using a random number generator. Those who were willing to participate were explained about the study purpose and informed consent was taken before the study. The subjects were provided with a proforma to obtain clinical and demographic information, details of COVID-19 testing and vaccination.
The study subjects were directed to the blood collection room and 3ml venous blood was drawn from each health-care worker in a sterile plain vacutainer under strict aseptic precautions. Serum was separated and stored at -20 °C until analysis. Quantitative determination of antibody was done by chemiluminescence immunoassay as per the manufacturer’s instructions, in a National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited lab.
The Elecsys Anti-SARS- CoV-2 assay was performed on Cobas e 411, e 601 and e 602 analyzers (Roche Diagnostics, Mannheim, Germany) for the in vitro quantitative determination of antibodies (Including IgG). The test principle was double antigen sandwich assay which used a recombinant protein representing the Receptor Binding Domain of the S antigen. In brief, the sample was incubated with the biotinylated recombinant antigen and the recombinant antigen labelled with ruthenium. This formed a sandwich complex. In the next step, streptavidin coated microparticles were added and the complex became bound to solid phase. The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of an electrode. Application of a voltage to the electrode induced chemiluminescent emission which was measured by a photomultiplier. Results were determined by a calibration curve and the analyte concentration of each sample was expressed in U/ml. Measuring range spanned from 0.40-250 U/ml. Values greater than or equal to 0.8 U/ml were considered positive and less than 0.8U/ml were considered negative. The sensitivity and specificity of the kit are 98.8% and 99.9 % respectively as per the manufacturer.
Numerical variables were expressed as mean and standard deviation. Categorical variables were expressed as frequency and percentage. The log transformed Antibody titer data were presented as geometric mean with 95% confidence level. Unpaired t test was utilized to assess two groups and ANOVA test was used to compare the differences among more than two group’s data on Antibody titer. The data was entered into Microsoft excel and analysed by using statistical software IBM SPSS Version 25. The p-value < 0.05 was considered as statistically significant.
Results
The study enrolled 187 health care workers who met the eligibility criteria. The data of 187 participants who received two doses of Covishield vaccine were included in the final statistical analysis. Of the 187 participants, 186 (99.47%) showed detectable Anti-SARS-CoV-2 antibody levels against the RBD protein. Values greater than or equal to 0.8 U/ml were considered positive and less than 0.8U/ml were considered negative. Lowest antibody titer was found to be 0.6U/ml and highest antibody titer was 5521U/ml. Table 1 shows mean, median and standard deviation of the total antibody level.
|
Table 1. Range of the total antibody level
 |
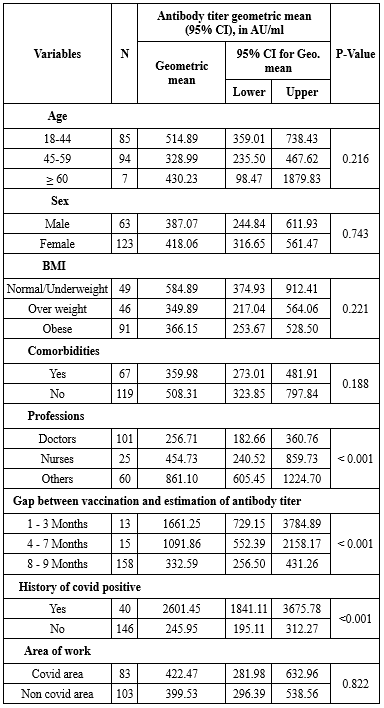
The baseline characteristics analyzed included age, gender, co-morbidities, area of work and BMI. Different categories of health-care workers were included in this study. Other parameters included were gap between vaccination and estimation of antibody titer as well as history of previous COVID-19 infection (Table 3).
The association between various factors and antibody titer was assessed (Table 4).
No significant correlation of antibody response was observed with age, gender, comorbidities, area of work (Whether worked in COVID ICUs and wards) and BMI (p-value>0.05).
The antibody response was found to be statistically significant among healthcare workers in various categories (p-value <0.001).
Antibody titer level was high within 3 months of vaccination and waning of titer was noticed as months passed by. This was also found to be significant. (p-value<0.001)
Those who had been previously infected with SARS-CoV-2 produced significantly higher antibody titer than those who were never infected. (p-value<0.001).
|
Table 4. Association between clinicodemographic characteristics and antibody titer
 |
Discussion
In the current cross-sectional study conducted among 187 health-care workers, who were administered two doses of Covishield vaccine, 99·47% developed Anti-SARS-CoV-2 antibodies including IgG against RBD protein. The seroconversion rates of most of the studies were greater than 95%. Hoque et al reported 100% of antispike protein IgG antibody (7). Seropositivity rate obtained in a study done by Singh et al was 98.1 % (4). Another study revealed 96.6% antibody response in their participants (8). Lower seropositivity (69.9%, 69.67%) was observed in the studies conducted by Mahadevaiah et al, Njarekkattuvalappil et al respectively (9,10).
Age, gender, comorbidities, area of work, body mass index, history of previous COVID infection, gap between vaccination and estimation of antibody titer were assessed as variables affecting antibody response.
No significant difference in antibody titer was seen in relation to age, gender, BMI, area of work and comorbid conditions. Singh et al obtained similar inference with regard to age, sex, BMI, and comorbidities (4). Balasubramanian et al couldn’t find significant association with age, sex and BMI (11). As per Njarekkattuvalappil et al BMI, gender and comorbidities were not found to be statistically significant (10). In contrast to the interpretation we made, a study done by Lustig et.al found that antibody responses were different in health care workers of varying age, gender, and comorbid conditions (12).
There is enough evidence that older individuals have reduced immune response (2,7,10,13,14). We were unable to ascertain this reality. Perhaps the very small number of participants over 60 in this cohort is the reason for this.
According to Uysal et al, the titers of anti-RBD antibodies were lower in obese participants compared to normal-weight participants. Excessive adiposity might have a negative impact on the immune system. Another presumed finding is that, as the ACE2 receptor is markedly seen in adipose tissue, obese individuals are more prone to get infection (15).
Similar to our findings, comorbidity did not make any difference in the SARSCoV-2 Ig G antibody level in other studies too (4,10,16). Since the health-care workers who participated in the study were with manageable comorbid conditions, we could not infer the association between antibody response and comorbidities.
In our study, different categories of health-care workers showed a significant correlation with the antibody response. The geometric mean titer was higher for nurses and other hospital staff compared to doctors. It could be due to the fact that a greater number of participants in these groups probably had asymptomatic infection with SARS COV-2. No difference in seroprevalence between various groups of health care workers were observed in serosurveillance done by Murhekar (17). Only limited studies are available regarding the correlation between post vaccination SARS-CoV2 antibodies and different categories of health-care workers.
The antibody titers of health-care workers who had previously been infected with SARS-CoV-2 were higher than those who were never infected; the association was found to be statistically significant. There are also other researchers who support our findings (11,16,18). The high antibody levels in the previously infected groups may be due to the fact that B cells create antibodies which multiply after each exposure, whether due to an infection or vaccination (18). Humans who have been infected with SARS-CoV-2 tend to produce long-lived bone marrow plasma cells, and the levels of serum anti-SARS-CoV-2 spike protein (S) antibodies remain detectable as long as 11 months post infection (19).
The level of antibodies in our participants decreased over time and their antibody titers were maximum within three months of vaccination. Waning of antibody responses to vaccines needs to be augmented by booster doses. Some other studies also noticed a decrease in antibody titer as time passed by (16,20,21). It is advisable that a follow-up study be conducted to determine the optimal timing for administration of booster dose to the previously infected group, by performing a regular serological analysis and monitoring the decline in antibody levels over time. The resulting data will help in developing strategies for vaccination as well as for developing protocols to prioritize individuals for booster shots (18).
Regarding limitations of this study, immunity against vaccination depends upon Neutralizing antibody titer and cell-mediated immune response which were not assessed. Effectiveness of booster dose could not be assessed.
Conclusion
There was a robust immune response in vaccine recipients irrespective of their age, gender, BMI, and comorbidity status. We could assess the antibody response after vaccination for different periods ranging from 1month to 9 months. SARS-CoV-2-infected healthcare workers had higher antibody titer than those who were not infected. Future studies are desirable to determine the protective level of antibody titer.
Acknowledgement
We would like to extend heartfelt gratitude to all the health-care workers of our hospital who took part in this study.
Funding sources
The authors received financial support from State board of medical research, under the head of account 2210-05-105-99-34-OC (Plan).
Ethical statement
The study was approved by the institutional Ethics committee (Reference no.IEC/GMC TSR/176/2021), Government Medical College, Thrissur, Kerala India.
Conflicts of interest
All authors declare no conflict of interest.
Author contributions
Concept and design of study were done by Kavitha Paul Konikkara and Reena John. Data collection was by Kavitha Paul Konikkara and Aiswarya Mukundan. Data analysis and drafting of the manuscript were carried out by Kavitha Paul Konikkara and Aiswarya Mukundan. Review and editing were by Reena John. Final approval of the version to be published were by Kavitha Paul Konikkara, Aiswarya Mukundan and Reena John.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Research Article: Original Paper |
Subject:
Microbiology
Received: 2023/05/8 | Accepted: 2023/11/26 | Published: 2025/04/18 | ePublished: 2025/04/18
Received: 2023/05/8 | Accepted: 2023/11/26 | Published: 2025/04/18 | ePublished: 2025/04/18
References
1. WHO COVID-19 Dashboard weekly update published on 23rd. 2022. [View at Publisher]
2. Jeewandara C, Kamaladasa A, Pushpakumara PD, Jayathilaka D, Aberathna IS, Rasikangani Danasekara DRS, et al. Immune responses to a single dose of the AZD1222/Covishield vaccine in health care workers. Nat commun 2021;12(1):4617. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Rafi AM, Tomy MM, Thomas R, Valsan C, Unnikrishnan UG, Innah SJ, et al. Serosurveillance of SARS-CoV-2 among the health-care Workers of a Tertiary Care Teaching Institution during the Post Lockdown Phase in Central Kerala, India. J Clin Diagn Res. 2021;15(7):13-7. [View at Publisher] [DOI] [Google Scholar]
4. Singh AK, Phatak SR, Sing R, Bhattacharjee K, Singh NK, Gupta A, et al. Antibody response after second dose of ChAdOx1-nCOV (Covishield TM) and BVB-125 (Covaxin TM) among health care workers in India: Final results of cross- sectional coronavirus vaccine-induced antibody titer (COVAT) study. Vaccine. 2021;39 (44): 6492-6509. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Subbarao S, Warrener LA, Hoschler K, Perry KR, Shute J, Whitaker H, et al. Robust antibody responses in 70-80-year-olds 3 weeks after the first or second doses of Pfizer/BioNTech COVID-19 vaccine, United Kingdom, January to February 2021. Euro Surveill. 2021;26(12):2100329 [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Lin D, Liu L, Zhang M, Hu Y, Yang Q, Guo J, et al. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. Eur J Clin Microbiol Infect Dis. 2020;39(12):2271-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Hoque A, Barshan AD, Chowdhury FUH, Fardous J, Hasan MJ, Saeed Khan Md A, et al. Antibody Response to ChAdOx1-nCoV-19 Vaccine Among Recipients in Bangladesh: A Prospective Observational Study. Infect Drug Resist. 2021;14:5491-500. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Haque MA, Tarik MH, Amin MR, et al. Antibody Response of Covishield Vaccine among Health Care Workers at Rajshahi Medical College Hospital- A Cross-Sectional Study. TAJ .2021;34:(2):01-08 [View at Publisher] [DOI] [Google Scholar]
9. Mahadevaiah A, Doddamadaiah C,KS S, Nanjappa MC. Study of immunogenicity, safety and efficacy of covishield vaccine among health care workers in a tertiary cardiac care centre. Indian J Med Microbiol 2022;40(2):200-3. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Njarekkattuvalappil SK, Bhaskaran R, Raj S, Jose P, Rafi AM, Thomas J, et al. Sero surveillance among health-care workers vaccinated with ChAdOx1 nCoV-19 Corona vaccine in a tertiary hospital of Kerala, India: Prospective cohort study. Sechenov Medical Journal. [View at Publisher] [DOI] [Google Scholar]
11. Balasubramanian R, Menon T. Antibody Response against Covid-19 among Vaccinated Health Care Workers - A Cross-Sectional Serosurveillance Study. Asian J Med Res. 2022;11(2):31-6. [View at Publisher]
12. Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999-1009. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6(9):1140-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Jabal KA, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from health-care workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6):2100096. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Uysal EB, Gümüş S, Bektöre B, Bozkurt H, Gözalan A. Evaluation of antibody response after COVID‐19 vaccination of health-care workers. J Med Virol. 2022;94(3):1060-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Bhuiyan TR, Akhtar M, Khaton F, Ara Rahman SI, Ferdous J, Alamgir ASM, et al. Covishield vaccine induces robust immune responses in Bangladeshi adults. IJID Reg. 2022;(3):211-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Murhekar MV, Bhatnagar T, Thangaraj JW, Saravanakumar V, Kumar MS, Selvaraju S, et al. SARS-CoV-2 seroprevalence among the general population and health-care workers in India, December 2020-January 2021. Int J Infect Dis. 2021;108:145-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Ali H, Alahmad B, Al-Shammari AA, Alterki A, Hammad M, Cherian P, et al. Previous COVID-19 Infection and Antibody Levels after Vaccination. Front public health 2021;9:778243. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Turner JS, Kim W, Kalaidina E, Goss CW, Rauseo AM, Schmitz AJ, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021;595(7867):421-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021;385(24):e84 [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Assaid N, Arich S, Charoute H, Akarid K, Ezzikouri S, Maaroufi A, et al. Anti-SARS-CoV-2 Antibody Responses 5 Months Post Complete Vaccination of Moroccan Health-care Workers. Vaccines 2022;10(3):465. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.