Volume 18, Issue 6 (Nov-Dec 2024)
mljgoums 2024, 18(6): 5-8 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rasheed B, Tawfeq B. The effect of methotrexate on blood, liver, and renal parameters in patients with rheumatoid arthritis. mljgoums 2024; 18 (6) :5-8
URL: http://mlj.goums.ac.ir/article-1-1848-en.html
URL: http://mlj.goums.ac.ir/article-1-1848-en.html
1- Department of Nursing, College of Health and Medical Techniques-Shekhan, Duhok Polytechnic University, Duhok, Iraq , bizav.naji@dpu.edu.krd
2- Medical Laboratory Sciences, College of Health Sciences, Duhok University, Iraq
2- Medical Laboratory Sciences, College of Health Sciences, Duhok University, Iraq
Keywords: C-Reactive protein, Aspartate aminotransferases, Alanine transaminase, Hemoglobins, Methotrexate, Rheumatoid factor
Full-Text [PDF 458 kb]
(1203 Downloads)
| Abstract (HTML) (6057 Views)
Discussion
According to EULAR guidelines, conventional synthetic Disease-Modifying Anti-Rheumatic Drugs (csDMARDs), particularly MTX in combination with low-dose glucocorticoids, should be used as the first-line treatment for RA. Despite being considered an essential therapy for RA, low-dose MTX therapy's exact mechanism of action and side effects are yet unknown (10). Changes in hemoglobin (Hb) and mean corpuscular volume (MCV) due to MTX treatment may influence red cell distribution width (RDW), as RDW is calculated based on MCV levels (11). In addition to biochemical analysis, which helps predict potential toxicities, clinicians prioritize managing adverse effects through clinical monitoring. However, no studies have identified a widespread occurrence of these disturbances in the general population. This gap in research motivated the present study, aiming to develop a more effective approach for improved patient care.
Furthermore, in this study, no significant increase in transaminase levels was observed at an MTX cumulative dose of 1.5 g. This finding suggests that RA patients included in the study may tolerate MTX better than psoriatic patients, which could explain the absence of notable liver enzyme elevations (15,16). Even though, there is minimal correlation between elevated concentration of transaminases and the likelihood of fibrosis and histological alterations (17,18). A meta-analysis of randomized controlled trials examined the risk of liver injury in MTX users. The study published in Seminars in Arthritis and Rheumatism, 45(2), 156-162, revealed that the frequency of raised liver enzyme concentrations during MTX treatment in patients with psoriasis was more than that of RA patients (14.5% vs. 7.5%) (19). Research on other populations indicates a modest association between cumulative MTX dose and fibrosis. Moreover, liver enzymes were particularly monitored among the biochemical markers examined during prolonged MTX treatment (20).
Additionally, a 50% dose reduction in the event that Creatinine clearance (CrCl) is less than 45 mL/min may result from a decrease in CrCl, which is another important factor in MTX-based treatment (21).
Effects of moderate renal insufficiency on pharmacokinetics of MTX in patients with RA (22). Renal disease in RA patients: The results of this study indicate that the cumulative MTX dose has a significant impact on renal function (p = 0.037 for creatinine and p = 0.036 for CrCl), particularly in female patients. A significantly higher prevalence of CrCl disturbances is also observed when compared to creatinine (13.18% vs. 1.09%, respectively, p <0.001), indicating that CrCl should be prioritized in renal monitoring (23). The results of another study indicate that the cumulative MTX dose has a significant impact on renal function (p = 0.037 for creatinine and p = 0.036 for CrCl), particularly in female patients. Considering renal function tests, creatinine levels were observed on days 1, 14, and 30, and the results were compared with those reported by (24). Similarly, in the evaluation of liver function tests, SGOT and SGPT levels were measured on days 1, 14, and 30, and the findings aligned with those of (25). The observed significant changes in GOT levels during treatment follow-up suggest that MTX may not always be toxic to the liver or kidneys when administered at low doses with proper clinical monitoring and investigations. Regarding serological tests, the current study found no significant differences in CRP levels between days 1st, 14th, and 30th, which is consistent with the findings of Ismaili et al. (2019) (26).
Conclusion
Methotrexate is the primary medication recommended for patients with RA, demonstrating significant effects on hematological, hepatic, and renal parameters. Although the administration of low-dose MTX on a weekly basis for autoimmune conditions may result in certain adverse effects, it is generally well-tolerated and exhibits considerable efficacy. Proper monitoring is essential to mitigate potential negative outcomes. Given that many of these serious complications can be detected early and potentially avoided, it is imperative for primary care physicians and hematologists to be aware of these issues and the associated guidelines. Prolonged MTX therapy may result in elevated liver enzyme levels. Serological assessments, such as CRP and RF tests, are critical for monitoring disease progression and the side effects of MTX. Additionally, conducting bone marrow examinations in relation to MTX treatment is advisable, as bone marrow suppression may provide valuable insights. If laboratory test abnormalities occur during treatment, it is recommended to reduce the methotrexate dosage or consider switching to an alternative medication with fewer side effects until the patient’s condition stabilizes.
Acknowledgement
We extend our deepest gratitude and praise to Almighty God for granting us the strength and wisdom to understand, learn, and successfully complete this research. We also sincerely thank the staff of the Duhok Rheumatology Center laboratory in Duhok City for their continuous support. A special appreciation goes to Baravan Amedi (Rheumatologist) for his valuable assistance in our study.
Funding sources
None.
Ethical statement
A questionnaire was taken from the participants after obtaining ethics approval from the Research Ethics Committee of Duhok Directorate of Health (13122023-11-17).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
BT: Data collection, filling questionnaire by asking patients, investigations, and drafting the manuscript; BR: Data collection, analysis of data, and drafting the manuscript.
Full-Text: (1101 Views)
Introduction
Rheumatoid arthritis (RA) is a chronic, symmetrical, inflammatory autoimmune disease that initially affects small joints, progressing to larger joints, and eventually the skin, eyes, heart, kidneys, and lungs. Often, the bone and cartilage of joints are destroyed, and tendons and ligaments weaken (1).
The onset of this disease is usually from the age of 35 to 60 years, with remission and exacerbation. It can also afflict young children even before the age of 16 years, referred to as juvenile RA (JRA), which is similar to RA except that rheumatoid factor (RF) is not found (2-4). In the West, the prevalence of RA is believed to be 1-2% (5,6), and 1% worldwide (7).
One of the most common screening procedures in rheumatologic therapies is liver enzyme testing, as recommendations, such as the widely used American College of Rheumatology guidelines, urge monitoring liver enzymes at intervals of at least 8-12 weeks in all RA patients treated with methotrexate (MTX) (8). Additionally, since MTX precipitates in the renal tubules and is primarily excreted by the kidneys, high doses used for oncological treatments may lead to acute kidney injury (AKI) (9). Therefore, close monitoring of renal function is essential when administering MTX.
Although low-dose MTX therapy is regarded as an anchor therapy in RA, full details of its mechanism of action and off-target effects are still incompletely understood (10).
Considering MTX’s effects on folic acid metabolism, MTX treatment can result in alterations of mean corpuscular volume (MCV), which may impact red cell distribution width (RDW), as MCV levels feed into RDW calculation (11). We thus questioned, whether RDW levels and subsequently its diagnostic utility and potential in RA subjects, as reported before, are influenced by ongoing MTX therapy (10,12-14). This study aimed to evaluate the effect of methotrexate on blood, liver, and renal parameters in patients with RA.
Methods
This cross-sectional study was conducted at Duhok Rheumatology Center from October 2023 to March 2024. Build statistics and a basis to standardize future practice and Hospital protocol.
Sixty consecutive patients aged 19-70 years with diagnosed RA on methotrexate treatment (10 mg) orally per week participated in this study. A questionnaire was taken from the participants after obtaining ethics approval from the Research Ethics Committee of Duhok Directorate of Health (13122023-11-17). Biochemical tests for renal and liver function, including creatinine, glutamic oxaloacetic transaminase (SGOT/AST), and glutamate pyruvate transaminase (SGPT/ALT), were performed alongside hematological tests such as complete blood count (CBC) and erythrocyte sedimentation rate (ESR). Additionally, serological tests, including C-reactive protein (CRP) and rheumatoid factor (RF), were conducted to monitor drug administration. A total of 6 mL of venous blood was collected from each participant, with 3 mL placed in an EDTA tube for hematological tests and the remaining 3 mL used for biochemical analysis. Instruments for data collection were as follows: Roche Cobas c 311 biochemical auto analyzer machine for serum CRP, SGOT, SGPT, and Creatinine levels, and Boule Medonic hematological auto analyzer machine for hemoglobin (Hb), white blood cell (WBC), and platelet (PLT) levels. Patients undergoing MTX treatment were included in the study, while those receiving anti-rheumatoid drug therapies other than MTX, as well as individuals with blood, renal, or liver diseases, were excluded.
Results
Age and hematological changes during 1st, 14th, and 30th days of the treatment
Age and hematological changes [Mean±Standard Deviation (SD)] were as follows: age (44.13±10.31) years, while Hb, WBC, PLT counts, and ESR on the first day of the treatment were 12.30±1.67 gm/dl, 8.85±2.68×109/L, 287.67±68.42×103/L, 31.37±20.19 mmHg/hr, respectively; on the day 14th of the treatment were 12.20±1.59, 8.79±2.65, 270.38±73.96, and 28.12±14.97, respectively; and on day 30th of the treatment were 12.20±1.92, 9.06 ±2.98, 278.38±35.96, and 26.86±17.03, respectively (Table 1).
Biochemical changes during 1st, 14th, and 30th days of the treatment
Also, biochemical changes in Creatinine, SGOT, and SGPT levels were as follows: on the first day of the treatment were 0.74±0.34, 24.38±16.76, and 25.58±17.26 gm/dl, respectively; on day 14th of the treatment were 0.71±0.32, 20.66±7.62, and 21.18±7.77 gm/dl, respectively; and on day thirty after treatment were 0.72±0.34, 20.32 ±8.11, and 20.53±8.07 gm/dl, respectively (Table 2).
Serological changes during 1st, 14th, and 30th days of the treatment
Serological changes in CRP and RF levels were as follows: on the first day of the treatment were 21.29±53.84 gm/dl and 76.20±77.15 U/ml, respectively; on day 14th of the treatment were 12.39±18.97 gm/dl and 44.46 ± 46.52 U/ml, respectively; on day thirty after treatment were 11.82 ±14.04 gm/dl, 43.26 ± 59.41 U/ml, respectively (Table 3).
Comparison of hematological changes during 1st, 14th, and 30th days of the treatment
There were no significant differences between Hb, WBC, and ESR on all treatment days (1st, 14th, and 30th) (p > 0.05), while the only significant differences in PLT levels were between 1st and 14th days (p < 0.05) (Table 4).
Comparison of biochemical changes during 1st, 14th, and 30th days of the treatment
There were no significant differences between Creatinine and SGOT on all treatment days (1st, 14th, and 30th) (p > 0.05), while the only significant differences in SGPT levels were between 1st and 30th days (p < 0.05) (Table 5).
Comparison of serological changes during 1st, 14th, and 30th days of the treatment:
There were no significant differences between CRP levels on all treatment days (1st, 14th, and 30th) (p > 0.05), while there were significant differences in RF levels between 1st and 14th days, as well as 1st and 30th days (p < 0.01 and p < 0.04, respectively) (Table 6).
Rheumatoid arthritis (RA) is a chronic, symmetrical, inflammatory autoimmune disease that initially affects small joints, progressing to larger joints, and eventually the skin, eyes, heart, kidneys, and lungs. Often, the bone and cartilage of joints are destroyed, and tendons and ligaments weaken (1).
The onset of this disease is usually from the age of 35 to 60 years, with remission and exacerbation. It can also afflict young children even before the age of 16 years, referred to as juvenile RA (JRA), which is similar to RA except that rheumatoid factor (RF) is not found (2-4). In the West, the prevalence of RA is believed to be 1-2% (5,6), and 1% worldwide (7).
One of the most common screening procedures in rheumatologic therapies is liver enzyme testing, as recommendations, such as the widely used American College of Rheumatology guidelines, urge monitoring liver enzymes at intervals of at least 8-12 weeks in all RA patients treated with methotrexate (MTX) (8). Additionally, since MTX precipitates in the renal tubules and is primarily excreted by the kidneys, high doses used for oncological treatments may lead to acute kidney injury (AKI) (9). Therefore, close monitoring of renal function is essential when administering MTX.
Although low-dose MTX therapy is regarded as an anchor therapy in RA, full details of its mechanism of action and off-target effects are still incompletely understood (10).
Considering MTX’s effects on folic acid metabolism, MTX treatment can result in alterations of mean corpuscular volume (MCV), which may impact red cell distribution width (RDW), as MCV levels feed into RDW calculation (11). We thus questioned, whether RDW levels and subsequently its diagnostic utility and potential in RA subjects, as reported before, are influenced by ongoing MTX therapy (10,12-14). This study aimed to evaluate the effect of methotrexate on blood, liver, and renal parameters in patients with RA.
Methods
This cross-sectional study was conducted at Duhok Rheumatology Center from October 2023 to March 2024. Build statistics and a basis to standardize future practice and Hospital protocol.
Sixty consecutive patients aged 19-70 years with diagnosed RA on methotrexate treatment (10 mg) orally per week participated in this study. A questionnaire was taken from the participants after obtaining ethics approval from the Research Ethics Committee of Duhok Directorate of Health (13122023-11-17). Biochemical tests for renal and liver function, including creatinine, glutamic oxaloacetic transaminase (SGOT/AST), and glutamate pyruvate transaminase (SGPT/ALT), were performed alongside hematological tests such as complete blood count (CBC) and erythrocyte sedimentation rate (ESR). Additionally, serological tests, including C-reactive protein (CRP) and rheumatoid factor (RF), were conducted to monitor drug administration. A total of 6 mL of venous blood was collected from each participant, with 3 mL placed in an EDTA tube for hematological tests and the remaining 3 mL used for biochemical analysis. Instruments for data collection were as follows: Roche Cobas c 311 biochemical auto analyzer machine for serum CRP, SGOT, SGPT, and Creatinine levels, and Boule Medonic hematological auto analyzer machine for hemoglobin (Hb), white blood cell (WBC), and platelet (PLT) levels. Patients undergoing MTX treatment were included in the study, while those receiving anti-rheumatoid drug therapies other than MTX, as well as individuals with blood, renal, or liver diseases, were excluded.
Results
Age and hematological changes during 1st, 14th, and 30th days of the treatment
Age and hematological changes [Mean±Standard Deviation (SD)] were as follows: age (44.13±10.31) years, while Hb, WBC, PLT counts, and ESR on the first day of the treatment were 12.30±1.67 gm/dl, 8.85±2.68×109/L, 287.67±68.42×103/L, 31.37±20.19 mmHg/hr, respectively; on the day 14th of the treatment were 12.20±1.59, 8.79±2.65, 270.38±73.96, and 28.12±14.97, respectively; and on day 30th of the treatment were 12.20±1.92, 9.06 ±2.98, 278.38±35.96, and 26.86±17.03, respectively (Table 1).
Biochemical changes during 1st, 14th, and 30th days of the treatment
Also, biochemical changes in Creatinine, SGOT, and SGPT levels were as follows: on the first day of the treatment were 0.74±0.34, 24.38±16.76, and 25.58±17.26 gm/dl, respectively; on day 14th of the treatment were 0.71±0.32, 20.66±7.62, and 21.18±7.77 gm/dl, respectively; and on day thirty after treatment were 0.72±0.34, 20.32 ±8.11, and 20.53±8.07 gm/dl, respectively (Table 2).
Serological changes during 1st, 14th, and 30th days of the treatment
Serological changes in CRP and RF levels were as follows: on the first day of the treatment were 21.29±53.84 gm/dl and 76.20±77.15 U/ml, respectively; on day 14th of the treatment were 12.39±18.97 gm/dl and 44.46 ± 46.52 U/ml, respectively; on day thirty after treatment were 11.82 ±14.04 gm/dl, 43.26 ± 59.41 U/ml, respectively (Table 3).
Comparison of hematological changes during 1st, 14th, and 30th days of the treatment
There were no significant differences between Hb, WBC, and ESR on all treatment days (1st, 14th, and 30th) (p > 0.05), while the only significant differences in PLT levels were between 1st and 14th days (p < 0.05) (Table 4).
Comparison of biochemical changes during 1st, 14th, and 30th days of the treatment
There were no significant differences between Creatinine and SGOT on all treatment days (1st, 14th, and 30th) (p > 0.05), while the only significant differences in SGPT levels were between 1st and 30th days (p < 0.05) (Table 5).
Comparison of serological changes during 1st, 14th, and 30th days of the treatment:
There were no significant differences between CRP levels on all treatment days (1st, 14th, and 30th) (p > 0.05), while there were significant differences in RF levels between 1st and 14th days, as well as 1st and 30th days (p < 0.01 and p < 0.04, respectively) (Table 6).
|
Table 1. Age and hematological changes during 1st, 14th, and 30th days of the treatment
 Table 2. Biochemical changes during 1st, 14th, and 30th days of the treatment 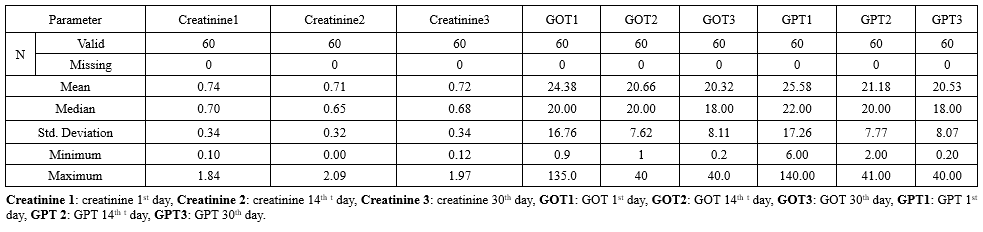 Table 3. Serological changes during 1st, 14th, and 30th days of the treatment  Table 4. Comparison of hematological changes during 1st, 14th, and 30th days of the treatment .PNG) |
Table 5. Comparison of biochemical changes during 1st, 14th, 30th days of the treatment.PNG) Table 6. Comparison of serological changes during 1st, 14th, and 30th days of the treatment .PNG) |
Discussion
According to EULAR guidelines, conventional synthetic Disease-Modifying Anti-Rheumatic Drugs (csDMARDs), particularly MTX in combination with low-dose glucocorticoids, should be used as the first-line treatment for RA. Despite being considered an essential therapy for RA, low-dose MTX therapy's exact mechanism of action and side effects are yet unknown (10). Changes in hemoglobin (Hb) and mean corpuscular volume (MCV) due to MTX treatment may influence red cell distribution width (RDW), as RDW is calculated based on MCV levels (11). In addition to biochemical analysis, which helps predict potential toxicities, clinicians prioritize managing adverse effects through clinical monitoring. However, no studies have identified a widespread occurrence of these disturbances in the general population. This gap in research motivated the present study, aiming to develop a more effective approach for improved patient care.
Furthermore, in this study, no significant increase in transaminase levels was observed at an MTX cumulative dose of 1.5 g. This finding suggests that RA patients included in the study may tolerate MTX better than psoriatic patients, which could explain the absence of notable liver enzyme elevations (15,16). Even though, there is minimal correlation between elevated concentration of transaminases and the likelihood of fibrosis and histological alterations (17,18). A meta-analysis of randomized controlled trials examined the risk of liver injury in MTX users. The study published in Seminars in Arthritis and Rheumatism, 45(2), 156-162, revealed that the frequency of raised liver enzyme concentrations during MTX treatment in patients with psoriasis was more than that of RA patients (14.5% vs. 7.5%) (19). Research on other populations indicates a modest association between cumulative MTX dose and fibrosis. Moreover, liver enzymes were particularly monitored among the biochemical markers examined during prolonged MTX treatment (20).
Additionally, a 50% dose reduction in the event that Creatinine clearance (CrCl) is less than 45 mL/min may result from a decrease in CrCl, which is another important factor in MTX-based treatment (21).
Effects of moderate renal insufficiency on pharmacokinetics of MTX in patients with RA (22). Renal disease in RA patients: The results of this study indicate that the cumulative MTX dose has a significant impact on renal function (p = 0.037 for creatinine and p = 0.036 for CrCl), particularly in female patients. A significantly higher prevalence of CrCl disturbances is also observed when compared to creatinine (13.18% vs. 1.09%, respectively, p <0.001), indicating that CrCl should be prioritized in renal monitoring (23). The results of another study indicate that the cumulative MTX dose has a significant impact on renal function (p = 0.037 for creatinine and p = 0.036 for CrCl), particularly in female patients. Considering renal function tests, creatinine levels were observed on days 1, 14, and 30, and the results were compared with those reported by (24). Similarly, in the evaluation of liver function tests, SGOT and SGPT levels were measured on days 1, 14, and 30, and the findings aligned with those of (25). The observed significant changes in GOT levels during treatment follow-up suggest that MTX may not always be toxic to the liver or kidneys when administered at low doses with proper clinical monitoring and investigations. Regarding serological tests, the current study found no significant differences in CRP levels between days 1st, 14th, and 30th, which is consistent with the findings of Ismaili et al. (2019) (26).
Conclusion
Methotrexate is the primary medication recommended for patients with RA, demonstrating significant effects on hematological, hepatic, and renal parameters. Although the administration of low-dose MTX on a weekly basis for autoimmune conditions may result in certain adverse effects, it is generally well-tolerated and exhibits considerable efficacy. Proper monitoring is essential to mitigate potential negative outcomes. Given that many of these serious complications can be detected early and potentially avoided, it is imperative for primary care physicians and hematologists to be aware of these issues and the associated guidelines. Prolonged MTX therapy may result in elevated liver enzyme levels. Serological assessments, such as CRP and RF tests, are critical for monitoring disease progression and the side effects of MTX. Additionally, conducting bone marrow examinations in relation to MTX treatment is advisable, as bone marrow suppression may provide valuable insights. If laboratory test abnormalities occur during treatment, it is recommended to reduce the methotrexate dosage or consider switching to an alternative medication with fewer side effects until the patient’s condition stabilizes.
Acknowledgement
We extend our deepest gratitude and praise to Almighty God for granting us the strength and wisdom to understand, learn, and successfully complete this research. We also sincerely thank the staff of the Duhok Rheumatology Center laboratory in Duhok City for their continuous support. A special appreciation goes to Baravan Amedi (Rheumatologist) for his valuable assistance in our study.
Funding sources
None.
Ethical statement
A questionnaire was taken from the participants after obtaining ethics approval from the Research Ethics Committee of Duhok Directorate of Health (13122023-11-17).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
BT: Data collection, filling questionnaire by asking patients, investigations, and drafting the manuscript; BR: Data collection, analysis of data, and drafting the manuscript.
Research Article: Original Paper |
Subject:
Laboratory Sciences
Received: 2024/08/3 | Accepted: 2024/12/31 | Published: 2025/04/26 | ePublished: 2025/04/26
Received: 2024/08/3 | Accepted: 2024/12/31 | Published: 2025/04/26 | ePublished: 2025/04/26
References
1. Lee JE, Kim IJ, Cho MS, Lee J. A case of rheumatoid vasculitis involving hepatic artery in early rheumatoid arthritis. Journal of Korean medical science. 2017; 32(7): 1207-10. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, Ansari RA, et al. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med Princ Pract. 2018; 27(6): 501-507. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New England Journal of Medicine. 2011; 365(23): 2205-19. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Chaudhari K, Rizvi S, Syed BA. Rheumatoid arthritis: current and future trends. Nat Rev Drug Discov. 2016; 15(5): 305-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Picerno V, Ferro F, Adinolfi A, Valentini E, Tani C, Alunno A. One year in review: the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2015; 33(4): 551-8. [View at Publisher] [PMID] [Google Scholar]
6. AlamanosY V. DrososAA. Incidence and Prevalence of Rheumatoid Arthritis, Based on the 1987 American College of Rheumatology Criteria: A Systematic Review. Semin Arthritis Rheum. 2006; 36(3): 182-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Chopra A, Abdel-Nasser A. Epidemiology of rheumatic musculoskeletal disorders in the developing world. Best Practice & Research Clinical Rheumatology. 2008; 22(4): 583-604. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Singh JA, Saag KG, Bridges SL, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016; 68(1): 1226‐26. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Hayashi K, Sada KE, Asano Y, Asano SH, Yamamura Y, Ohashi K, et al. Risk of higher dose methotrexate for renal impairment in patients with rheumatoid arthritis. Sci Rep. 2020; 10(1): 18715.
https://doi.org/10.1038/s41598-020-75655-9 [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nature Reviews Rheumatology. 2016; 12(12): 731-42. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. Journal of the American College of Cardiology. 2007; 50(1): 40-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Ballina-García FJ, Suárez A. Red cell distribution width is associated with cardiovascular risk and disease parameters in rheumatoid arthritis. Rheumatology. 2015; 54(4): 641-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Weiss G. Monitoring iron therapy in chronic heart failure. European journal of heart failure. 2013; 15(7): 711-2. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Al Taii H, Yaqoob Z, Al-Kindi SG. Red cell distribution width (RDW) is associated with cardiovascular disease risk in Crohn's disease. Clinics and research in hepatology and gastroenterology. 2017; 41(4): 490-2. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Hassan W. Methotrexate and liver toxicity: role of surveillance liver biopsy. Conflict between guidelines for rheumatologists and dermatologists. Annals of the rheumatic diseases. 1996; 55(5): 273. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Gilani ST, Khan DA, Khan FA, Ahmed M. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J Coll Physicians Surg Pak. 2012; 22(2): 101-4. [View at Publisher] [DOI] [Google Scholar]
17. Whiting-O'Keefe QE, Fye KH, Sack KD. Methotrexate and histologic hepatic abnormalities: a meta-analysis. The American journal of medicine. 1991; 90(6): 711-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Conway R, Low C, Coughlan RJ, O'Donnell MJ, Carey JJ. Methotrexate use and risk of lung disease in psoriasis, psoriatic arthritis, and inflammatory bowel disease: systematic literature review and meta-analysis of randomised controlled trials. bmj. 2015; 350. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Tilling L, Townsend S, David J. Methotrexate and hepatic toxicity in rheumatoid arthritis and psoriatic arthritis. Clinical drug investigation. 2006; 26: 55-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Kim TY, Kim JY, Sohn JH, Lee HS, Bang SY, Kim Y, et al. Assessment of substantial liver fibrosis by real‐time shear wave elastography in methotrexate‐treated patients with rheumatoid arthritis. Journal of Ultrasound in Medicine. 2015; 34(9): 1621-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Bressolle F, Bologna C, Kinowski JM, Sany J, Combe B. Effects of moderate renal insufficiency on pharmacokinetics of methotrexate in rheumatoid arthritis patients. Annals of the rheumatic diseases. 1998; 57(2): 110-3. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Karie S, Gandjbakhch F, Janus N, Launay-Vacher V, Rozenberg S, Mai Ba CU, Bourgeois P, Deray G. Kidney disease in RA patients: prevalence and implication on RA-related drugs management: the MATRIX study. Rheumatology. 2008; 47(3): 350-4. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Seideman P, Müller-Suur R. Renal effects of aspirin and low dose methotrexate in rheumatoid arthritis. Annals of the rheumatic diseases. 1993; 52(8): 613-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Kremer JM, Petrillo GF, Hamilton RA. Pharmacokinetics and renal function in patients with rheumatoid arthritis receiving a standard dose of oral weekly methotrexate: association with significant decreases in creatinine clearance and renal clearance of the drug after 6 months of therapy. J Rheumatol. 1995; 22(1): 38-40. [View at Publisher] [PMID] [Google Scholar]
25. Karlsson Sundbaum J, Eriksson N, Hallberg P, Lehto N, Wadelius M, Baecklund E. Methotrexate treatment in rheumatoid arthritis and elevated liver enzymes: A long-term follow-up of predictors, surveillance, and outcome in clinical practice. International journal of rheumatic diseases. 2019; 22(7): 1226-1232. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Ismaili H, Ismaili L, Rexhepi M. Values and Correlations between C-Reactive Protein and Apolipoprotein B after Treatment with Methotrexate at Patients with Rheumatoid Arthritis. Open Access Maced J Med Sci. 2019; 7(8): 1293-1298. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com